Trinitite
Created July 16, 1945 at the Trinity Atomic Test Site
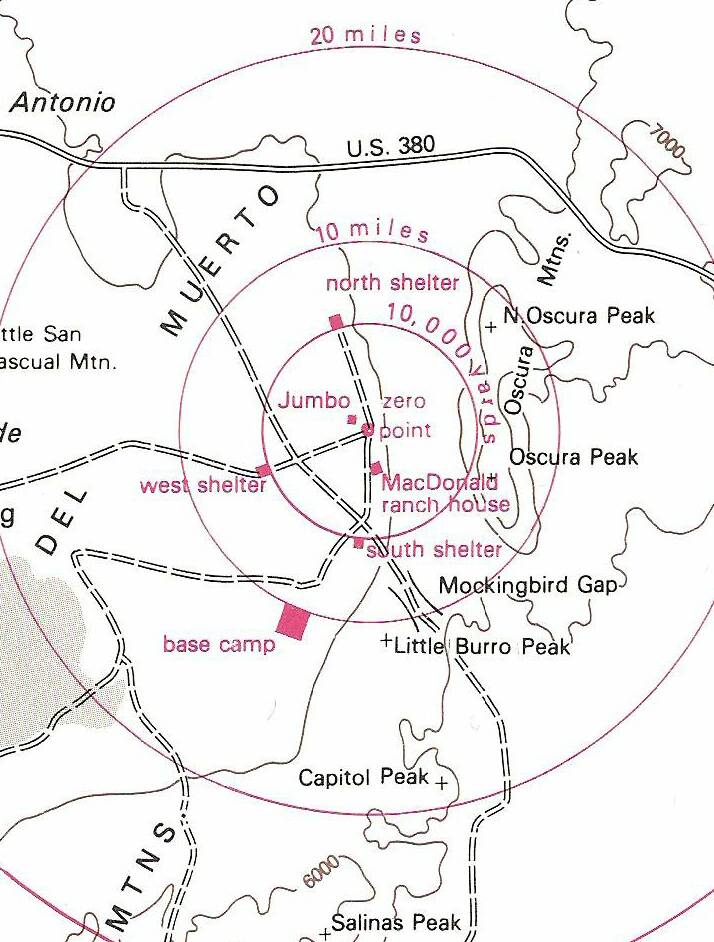
White Sands Missile Range
Jornada del Muerto Valley
Tularosa Basin
Alamogordo
Socorro County
NEW MEXICO
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Trinitite, also known as atomsite or Alamogordo glass, is the glassy residue left on the desert floor after the plutonium-based Trinity nuclear bomb test on July 16, 1945, near Alamogordo, New Mexico. The glass is primarily composed of arkosic sand composed of quartz grains and feldspar (both microcline and smaller amount of plagioclase with small amount of calcite, hornblende and augite in a matrix of sandy clay) that was melted by the atomic blast. It is usually a light green, although color can vary. It is mildly radioactive but safe to handle.
In the late 1940s and early 1950s, samples were gathered and sold to mineral collectors as a novelty. Traces of the material may be found at the Trinity Site today, although most of it was bulldozed and buried by the United States Atomic Energy Commission in 1953. It is now illegal to collect the remaining material from the site; however, material that was taken prior to this prohibition is still in the hands of collectors.
In 2005 it was theorized by Los Alamos National Laboratory scientist Robert Hermes and independent investigator William Strickfaden that much of the mineral was formed by sand which was drawn up inside the fireball itself and then rained down in a liquid form. In a 2010 article in Geology Today, Nelson Eby of the University of Massachusetts at Lowell and Robert Hermes described Trinitite as follows:
The glass has been described as a layer 1 to 2 centimeters thick, with the upper surface marked by a very thin sprinkling of dust which fell upon it while it was still molten. At the bottom is a thicker film of partially fused material, which grades into the soil from which it was derived. The color of the glass is a pale bottle green, and the material is extremely vesicular with the size of the bubbles ranging to nearly the full thickness of the specimen.
An estimated 4.3 Terajoules of heat energy went into forming the glass. The temperature required to melt the sand into the glass form observed is about 1470 C. Hence this is the estimated minimum temperature to which the sand was heated.
Contained within the glass are melted bits of the first atomic bomb and the support structures and various radionuclides formed during the detonation. The glass itself is marvelously complex at the tens to hundreds of micrometers scale, and besides glasses of varying composition also contains unmelted quartz grains. Air transport of the melted material led to the formation of spheres and dumbbell shaped glass particles. Similar glasses are formed during all ground level nuclear detonations and contain forensic information that can be used to identify the atomic device.
Trinity Test Site Map [1945]
The Trinity Explosion [July 16, 1945]
The Trinity explosion, 16 ms after detonation.
The viewed hemisphere's highest point in this image is about 200 meters (660 ft) high.
The Trinity Explosion [July 16, 1945]
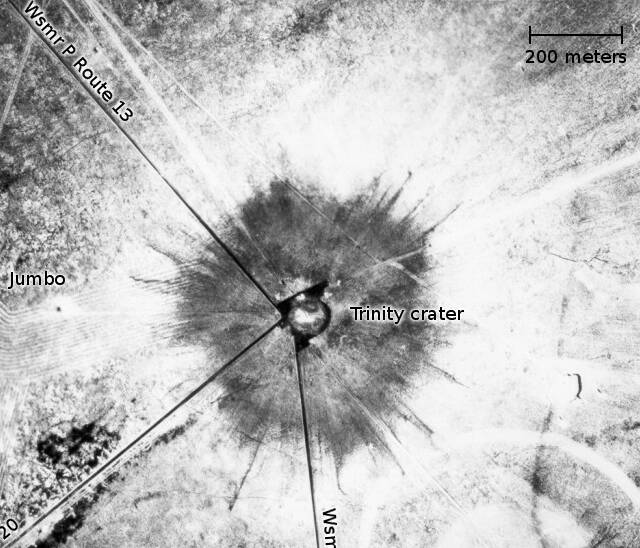
Aerial View of Trinity Crater [1945]
Trinity Ground Zero [1945]
Photographed by Michael P. Klimetz
Trinitite Grains
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
4.0 cm
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz
Photographed by Michael P. Klimetz